人工智能用于风险分层:多模态深度学习模型为肺栓塞提供增强预后
链接表
摘要
- 引言
- 方法
- 结果
- 讨论
- 结论、致谢和参考文献
5. 结论
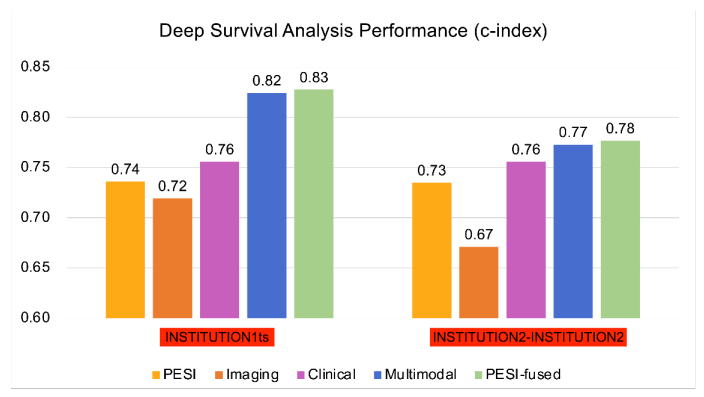
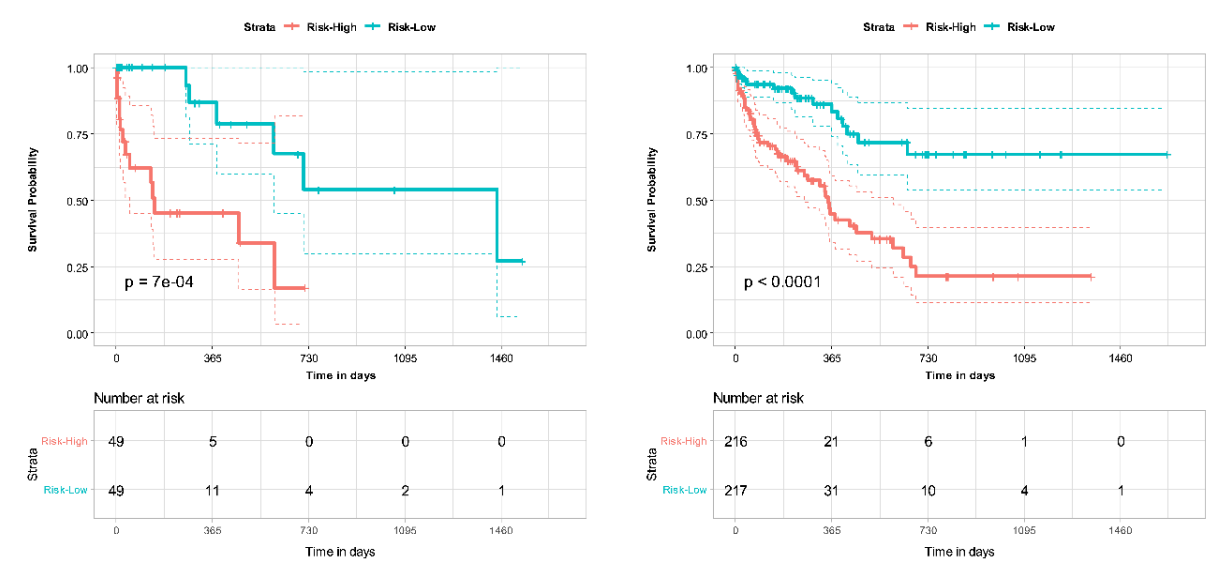
基于CTPA特征和临床变量组合的多组学深度学习模型在肺栓塞死亡率预测方面表现优于单独使用PESI评分。将PESI添加到多模态模型中仅显示出微小的性能改进,说明基于人工智能的模型已足够胜任生存预测。多模态模型在30天死亡风险估计方面同样优于单独使用PESI。通过NRI分析,临床和影像数据被独立证明都有助于提高多模态模型的性能。这些发现展示了多模态深度学习模型相比当前临床标准PESI的优势,将预后转变为一个整合更多临床和影像信息的智能过程。此外,我们证明了我们的模型与临床死亡率指标(如右心室功能障碍)的一致性。进一步分析可以更清楚地揭示肺栓塞患者各种风险因素与死亡率之间的联系,以及如何利用这些信息进行生存预测模型开发。然而,我们模型的益处只能通过在更大更多样化的数据集上进行额外验证以及对开发模型的前瞻性测试来确认。
\ 我们的研究强调了基于深度学习模型在肺栓塞患者预后和风险分层中的实用性。人工智能有潜力通过提供快速准确的诊断和预后信息来改善放射科医师和临床医生的工作流程。通过为肺栓塞患者提供及时且准确的风险分层,人工智能可能通过指导临床决策为患者和医疗提供者带来实质性益处,从而潜在地改善患者预后。
致谢
无。
参考文献
-
Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. American journal of preventive medicine. 2010;38(4):S495-S501.
\
-
Lewis AE, Gerstein NS, Venkataramani R, Ramakrishna H. Evolving management trends and outcomes in catheter management of acute pulmonary embolism. Journal of Cardiothoracic and Vascular Anesthesia. 2022;36(8):3344-3356.
\
-
Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. The Lancet. 2012;379(9828):1835-1846.
\
-
Piazza G, Goldhaber SZ. Acute pulmonary embolism: part I: epidemiology and diagnosis. Circulation. 2006;114(2):e28-e32.
\
-
Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. American journal of respiratory and critical care medicine. 2005;172(8):1041-1046.
\
-
Donzé J, Le Gal G, Fine MJ, et al. Prospective validation of the pulmonary embolism severity index. Thrombosis and haemostasis. 2008;100(05):943-948.
\
-
Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. 2008;
\
-
Fox J, Weisberg S. Cox proportional-hazards regression for survival data. An R and S-PLUS companion to applied regression. 2002;2002
\
-
Katzman JL, Shaham U, Cloninger A, Bates J, Jiang T, Kluger Y. DeepSurv: personalized treatment recommender system using a Cox proportional hazards deep neural network. BMC medical research methodology. 2018;18(1):1-12.
\
-
Oren O, Gersh BJ, Bhatt DL. Artificial intelligence in medical imaging: switching from radiographic pathological data to clinically meaningful endpoints. The Lancet Digital Health. 2020;2(9):e486-e488.
\
-
Weikert T, Winkel DJ, Bremerich J, et al. Automated detection of pulmonary embolism in CT pulmonary angiograms using an AI-powered algorithm. European radiology. 2020;30:6545-6553.
\
-
Huang S-C, Kothari T, Banerjee I, et al. PENet—a scalable deep-learning model for automated diagnosis of pulmonary embolism using volumetric CT imaging. NPJ digital medicine. 2020;3(1):61.
\
-
Huang S-C, Pareek A, Zamanian R, Banerjee I, Lungren MP. Multimodal fusion with deep neural networks for leveraging CT imaging and electronic health record: a case-study in pulmonary embolism detection. Scientific reports. 2020;10(1):22147.
\
-
Liu W, Liu M, Guo X, et al. Evaluation of acute pulmonary embolism and clot burden on CTPA with deep learning. European radiology. 2020;30:3567-3575.
\
-
Yao J, Zhu X, Zhu F, Huang J. Deep correlational learning for survival prediction from multi-modality data. Springer; 2017:406-414.
\
-
Vale-Silva LA, Rohr K. Long-term cancer survival prediction using multimodal deep learning. Scientific Reports. 2021;11(1):13505.
\
-
Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. New England Journal of Medicine. 2014;370(15):1402-1411.
\
-
Hofmanninger J, Prayer F, Pan J, Röhrich S, Prosch H, Langs G. Automatic lung segmentation in routine imaging is primarily a data diversity problem, not a methodology problem. European Radiology Experimental. 2020;4(1):1-13.
\
-
Harrell Jr FE, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Statistics in medicine. 1984;3(2):143-152.
\
-
Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Annals of internal medicine. 2014;160(2):122-131.
\
-
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American statistical association. 1958;53(282):457-481.
\
-
Grifoni S, Olivotto I, Cecchini P, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101(24):2817-2822.
\
-
Coutance G, Cauderlier E, Ehtisham J, Hamon M, Hamon M. The prognostic value of markers of right ventricular dysfunction in pulmonary embolism: a meta-analysis. Critical care. 2011;15:1-10.
\
-
Chaudhary K, Poirion OB, Lu L, Garmire LX. Deep learning–based multi-omics integration robustly predicts survival in liver cancer. Clinical Cancer Research. 2018;24(6):1248-1259.
\
-
Somani SS, Honarvar H, Narula S, et al. Development of a machine learning model using electrocardiogram signals to improve acute pulmonary embolism screening. European Heart Journal-Digital Health. 2022;3(1):56-66.
\
-
Tourassi GD, Floyd CE, Sostman HD, Coleman RE. Acute pulmonary embolism: artificial neural network approach for diagnosis. Radiology. 1993;189(2):555-558.
\
-
Soffer S, Klang E, Shimon O, et al. Deep learning for pulmonary embolism detection on computed tomography pulmonary angiogram: a systematic review and meta-analysis. Scientific reports. 2021;11(1):15814.
\
-
Elias A, Mallett S, Daoud-Elias M, Poggi J-N, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ open. 2016;6(4):e010324.
\
-
Cahan N, Klang E, Marom EM, et al. Multimodal fusion models for pulmonary embolism mortality prediction. Scientific Reports. 2023;13(1):1-15.
图表
\ 
\ 
\ 
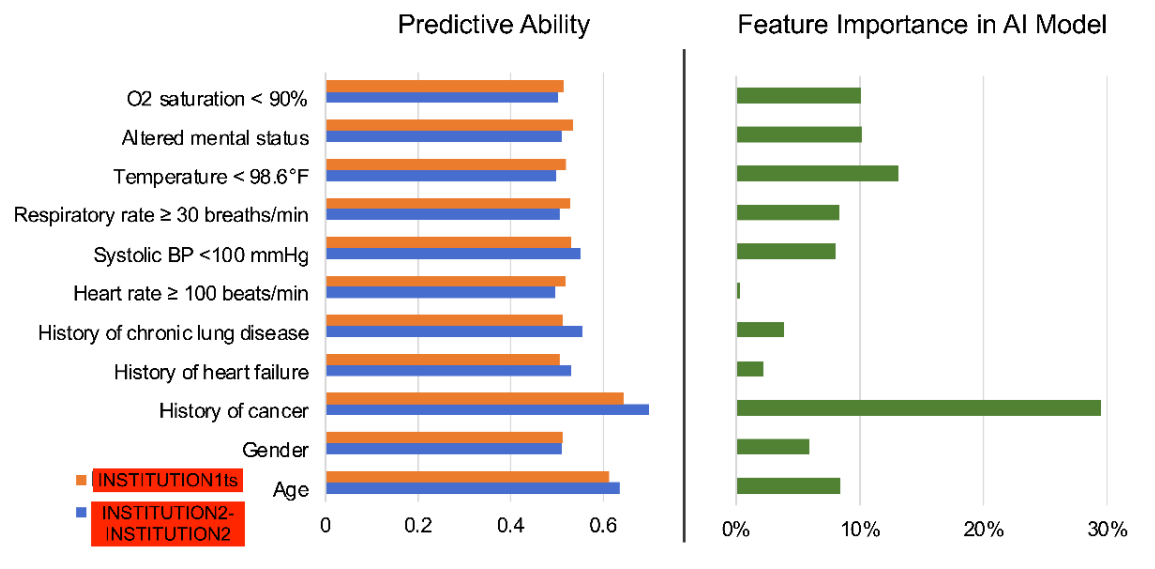
\ 
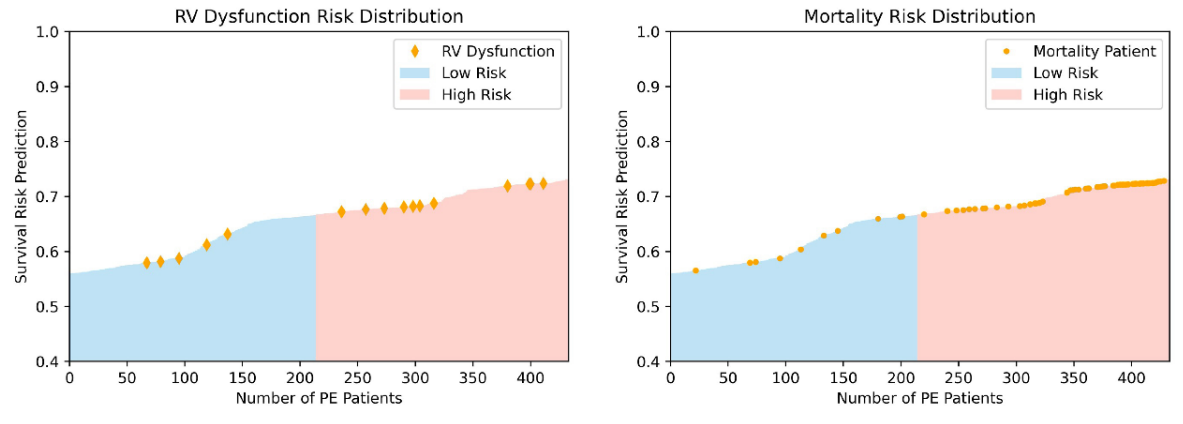
\ 
\ 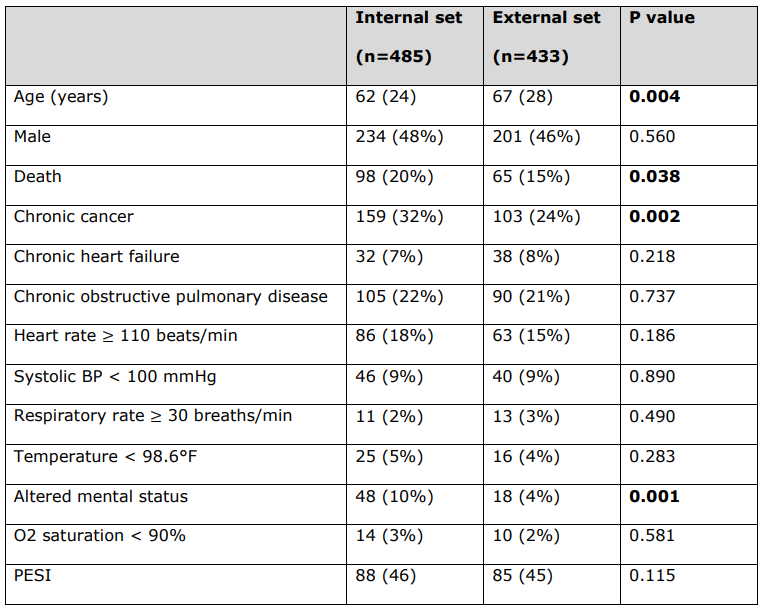
\ 用于计算每位患者PESI评分的PESI临床变量的详细患者特征。
\ 所有连续变量均报告为中位数(四分位距),所有分类变量均报告为数量(%)。统计学显著的p值以粗体显示(p < 0.05)。死亡状态不是PESI临床变量。
\ BP = 血压。PESI = 肺栓塞严重程度指数。
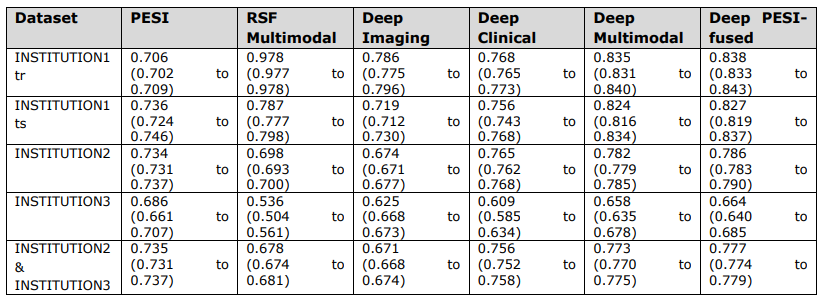
\ 

您可能也会喜欢

以太坊再定价:从 Rollup-Centric 到“安全性结算层”

Robinhood报告创纪录的季度收入,尽管加密货币市场低迷
